Learn About Why RU-58841 Did Not Receive FDA Approval Despite Results
In the realm of hair loss treatments, the search for effective solutions is relentless, with millions worldwide seeking remedies to restore their luscious locks. Among the myriad of potential treatments explored, RU-58841 emerged as a promising candidate, hailed for its anti-androgenic properties and potential to combat hair loss. However, despite initial enthusiasm and hopeful expectations, RU-58841 faced a significant hurdle: the lack of approval from the U.S. Food and Drug Administration (FDA). In this article, we delve into the intricacies surrounding RU-58841’s journey and uncover the underlying reasons why it fell short of FDA approval for treating hair loss. Through this exploration, we aim to shed light on the challenges and complexities inherent in the regulatory process, as well as the implications for individuals seeking effective treatments for hair loss.
WHAT IS RU-58841?
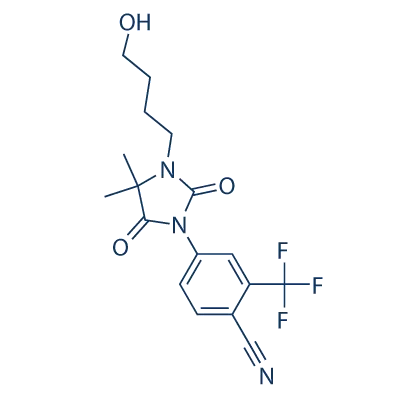
RU-58841, a non-steroidal anti-androgen compound, garnered attention in the realm of hair loss treatment due to its potential to address androgenetic alopecia, commonly known as male-pattern baldness and female-pattern hair loss. Unlike some traditional treatments like minoxidil and finasteride, which work by promoting hair growth or inhibiting the production of dihydrotestosterone (DHT), RU-58841 operates by directly blocking the androgen receptors in the scalp. By doing so, it aims to counteract the effects of DHT, a hormone associated with hair follicle miniaturization and eventual hair loss in individuals genetically predisposed to androgenetic alopecia.
RU-58841’s mechanism of action holds promise for individuals seeking alternatives to conventional hair loss treatments, particularly those who may experience side effects or limited efficacy with existing options. Additionally, its non-steroidal nature distinguishes it from some other anti-androgen medications, potentially reducing the risk of systemic side effects often associated with hormonal treatments. While its exact mode of action and long-term effects warrant further research, RU-58841 has sparked interest as a novel approach to addressing the underlying hormonal factors contributing to hair loss. However, its journey to regulatory approval and widespread use faces challenges and uncertainties, underscoring the complexities inherent in the development of new treatments for hair loss.
BACKGROUND OF RU-58841
RU-58841 emerged from the realm of hair loss research as a potential game-changer in the treatment landscape, offering a novel approach to addressing the underlying hormonal factors associated with androgenetic alopecia. Developed as a non-steroidal anti-androgen compound, RU-58841 aimed to disrupt the binding of dihydrotestosterone (DHT) to androgen receptors in the scalp, thereby thwarting the hormonal cascade that leads to hair follicle miniaturization and eventual hair loss. The compound’s unique mechanism of action sparked interest among researchers and pharmaceutical companies alike, setting the stage for its exploration as a potential treatment for hair loss.
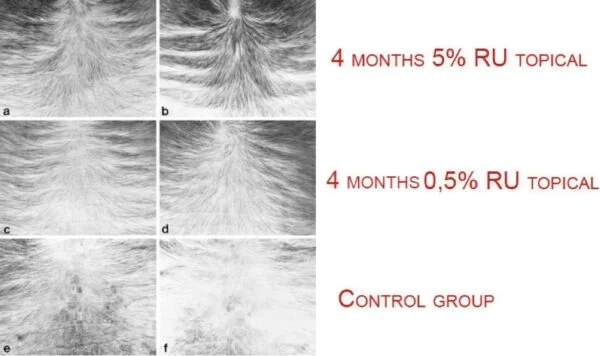
In the early stages of its development, RU-58841 underwent rigorous preclinical studies to evaluate its safety, efficacy, and mechanism of action. These studies, conducted primarily in laboratory settings and animal models, provided valuable insights into the compound’s pharmacological properties and its potential for hair growth promotion. Encouraged by promising preclinical results, researchers proceeded to initiate early clinical trials to assess RU-58841’s performance in human subjects with androgenetic alopecia. These trials aimed to evaluate the compound’s tolerability, safety profile, and preliminary efficacy in stimulating hair growth and halting further hair loss.
The initial promise of RU-58841 as a treatment for hair loss stemmed from early clinical findings suggesting its potential to mitigate the effects of androgens on the scalp and promote hair regrowth in individuals with androgenetic alopecia. These early studies hinted at RU-58841’s ability to target the root cause of hair loss by blocking androgen receptors, offering hope for a more targeted and effective treatment approach. As a result, pharmaceutical companies and researchers pursued further investigation into RU-58841’s potential, fueling optimism within the hair loss community and sparking anticipation for a breakthrough treatment option. However, despite the initial excitement surrounding RU-58841, its journey toward regulatory approval and widespread use faced significant challenges and uncertainties, underscoring the complexities inherent in the development of new treatments for hair loss.
REASONS FOR LACK OF FDA APPROVAL
The journey of RU-58841 towards FDA approval for hair loss treatment was fraught with obstacles that ultimately led to its failure to secure regulatory clearance. One primary reason for the lack of FDA approval was the insufficient clinical evidence supporting its safety and efficacy. Despite promising preclinical and early clinical trial results, there was a lack of large-scale, well-designed clinical trials to conclusively demonstrate RU-58841’s benefits in treating hair loss. Without robust clinical data, regulatory authorities such as the FDA were understandably hesitant to approve the drug for widespread use.
Safety concerns also played a significant role in the FDA’s decision-making process regarding RU-58841. While the compound showed potential as an anti-androgen treatment for hair loss, there were uncertainties regarding its long-term safety profile and potential side effects. Without comprehensive data on the compound’s safety, regulators were unwilling to approve RU-58841 for use in treating hair loss, prioritizing patient safety above all else.
Limited research and development efforts further hindered RU-58841’s path to FDA approval. The pharmaceutical companies or research institutions responsible for developing RU-58841 may have faced challenges such as funding constraints, resource limitations, or strategic shifts in priorities that impeded their ability to conduct further research and advance the drug through the regulatory approval process. Without continued investment and commitment to research and development, RU-58841 remained stagnant in its development journey, unable to overcome the hurdles blocking its path to approval.
Additionally, regulatory hurdles and challenges inherent in the FDA approval process likely contributed to the lack of approval for RU-58841. The regulatory pathway for new pharmaceuticals is complex and rigorous, requiring extensive documentation, data, and compliance with regulatory standards. If RU-58841 encountered obstacles or delays in meeting these requirements, it would have hampered its progress towards FDA approval, further prolonging its journey or leading to its eventual abandonment as a hair loss treatment option. Overall, the combination of insufficient clinical evidence, safety concerns, limited research and development, and regulatory hurdles ultimately prevented RU-58841 from obtaining FDA approval for treating hair loss.
CONCLUSION
The journey of RU-58841 towards FDA approval for hair loss treatment underscores the complexities and challenges inherent in the development of new pharmaceuticals. Despite its initial promise and potential as a novel anti-androgenic compound for combating hair loss, RU-58841 ultimately fell short of regulatory approval due to several key factors. Insufficient clinical evidence, safety concerns, limited research and development efforts, and regulatory hurdles collectively hindered RU-58841’s progress towards FDA approval, highlighting the rigorous standards and stringent criteria set forth by regulatory authorities to ensure the safety and efficacy of pharmaceutical interventions. While the lack of FDA approval represents a setback for RU-58841 and the broader field of hair loss treatment, it also serves as a reminder of the importance of robust clinical research, rigorous safety evaluation, and strategic investment in drug development. Moving forward, continued innovation, collaboration, and regulatory oversight will be essential in advancing the development of safe and effective treatments for hair loss and other medical conditions.
SHOP FOR HAIR LOSS TREATMENTS